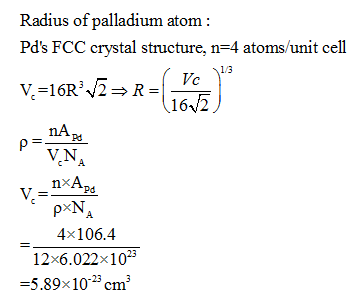
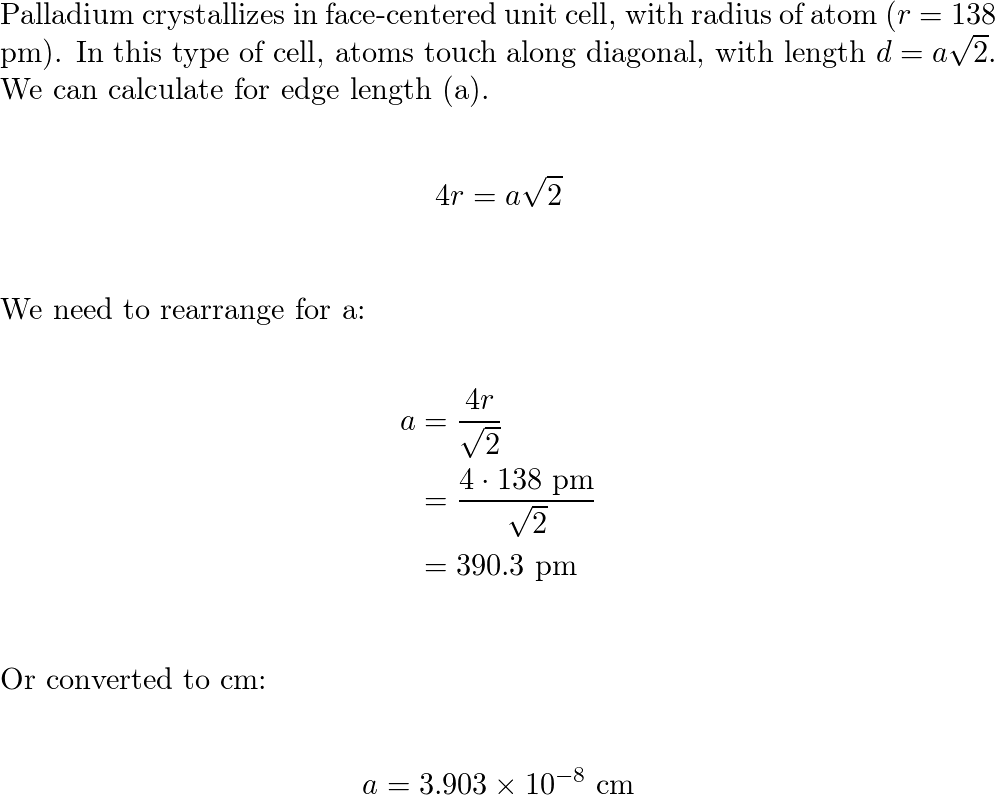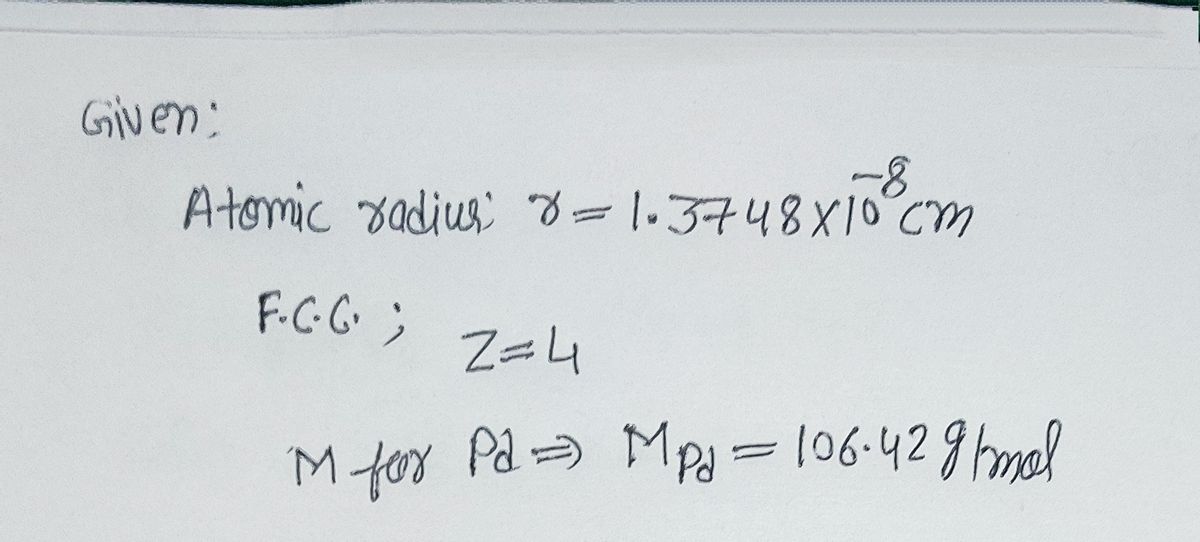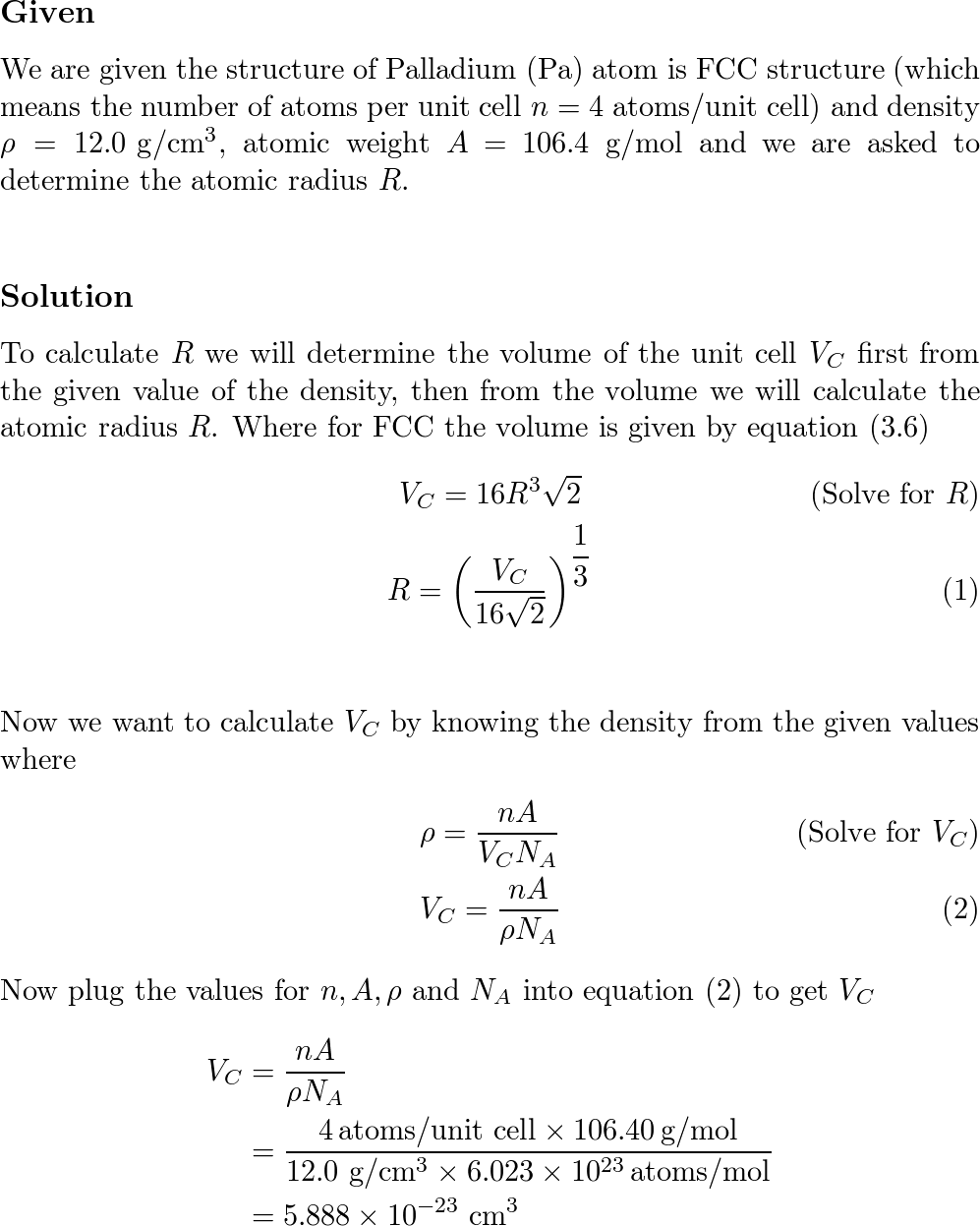HW2 answer(1) - 3.9 Calculate the radius of a palladium (Pd) atom, given that Pd has an FCC crystal structure, a density of 12.0 g/cm3, and an atomic | Course Hero

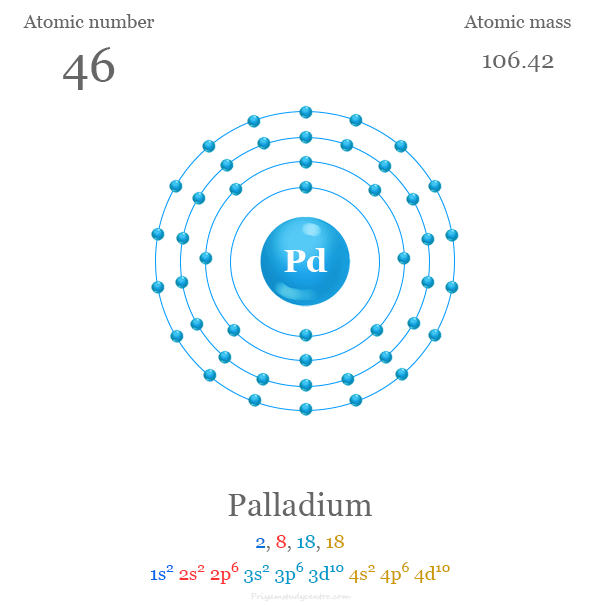
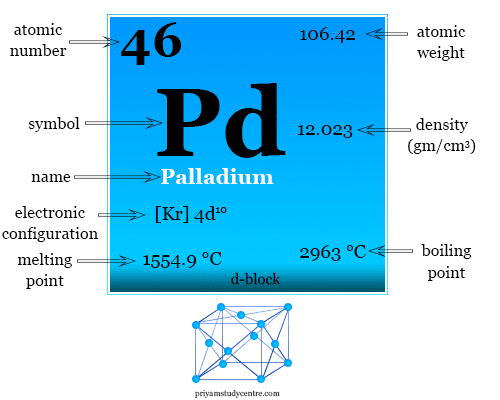
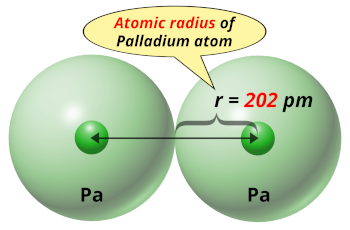
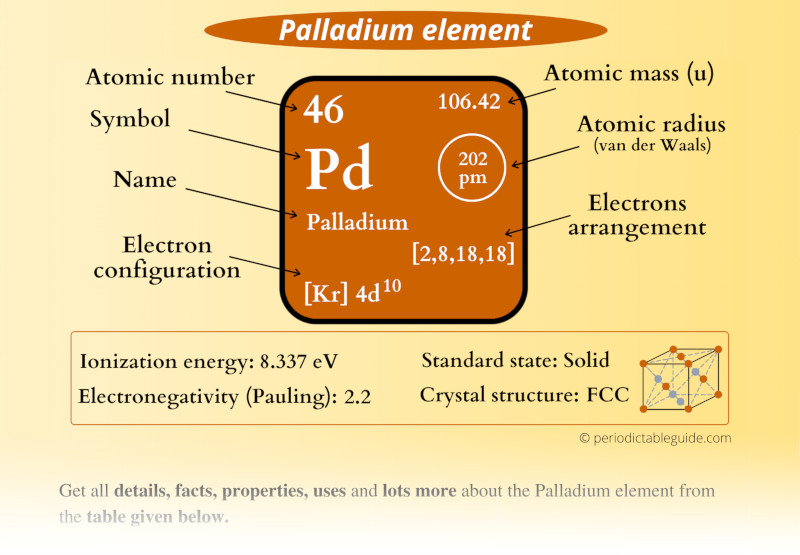
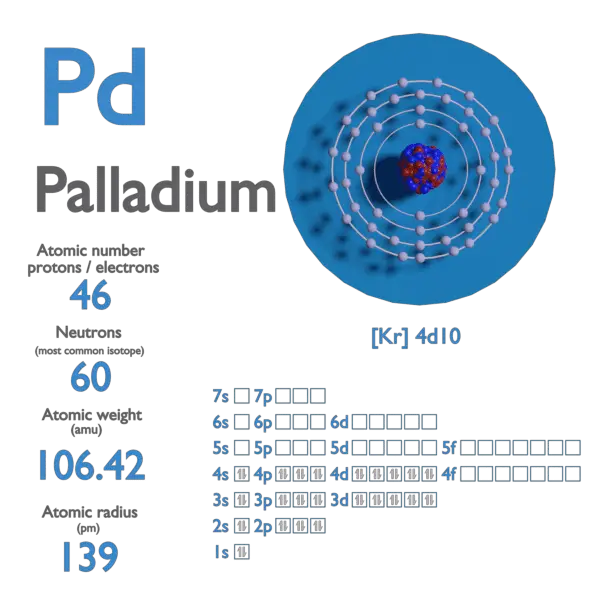
Pd Palladium Element Information: Facts, Properties, Trends, Uses and comparison - Periodic Table of the Elements | SchoolMyKids

SOLVED: Calculate the radius of a palladium atom in nm, given that Pd has an FCC crystal structure, a density of 12.0 g/cm3 and an atomic weight of 106.4 g/mol. A. 138
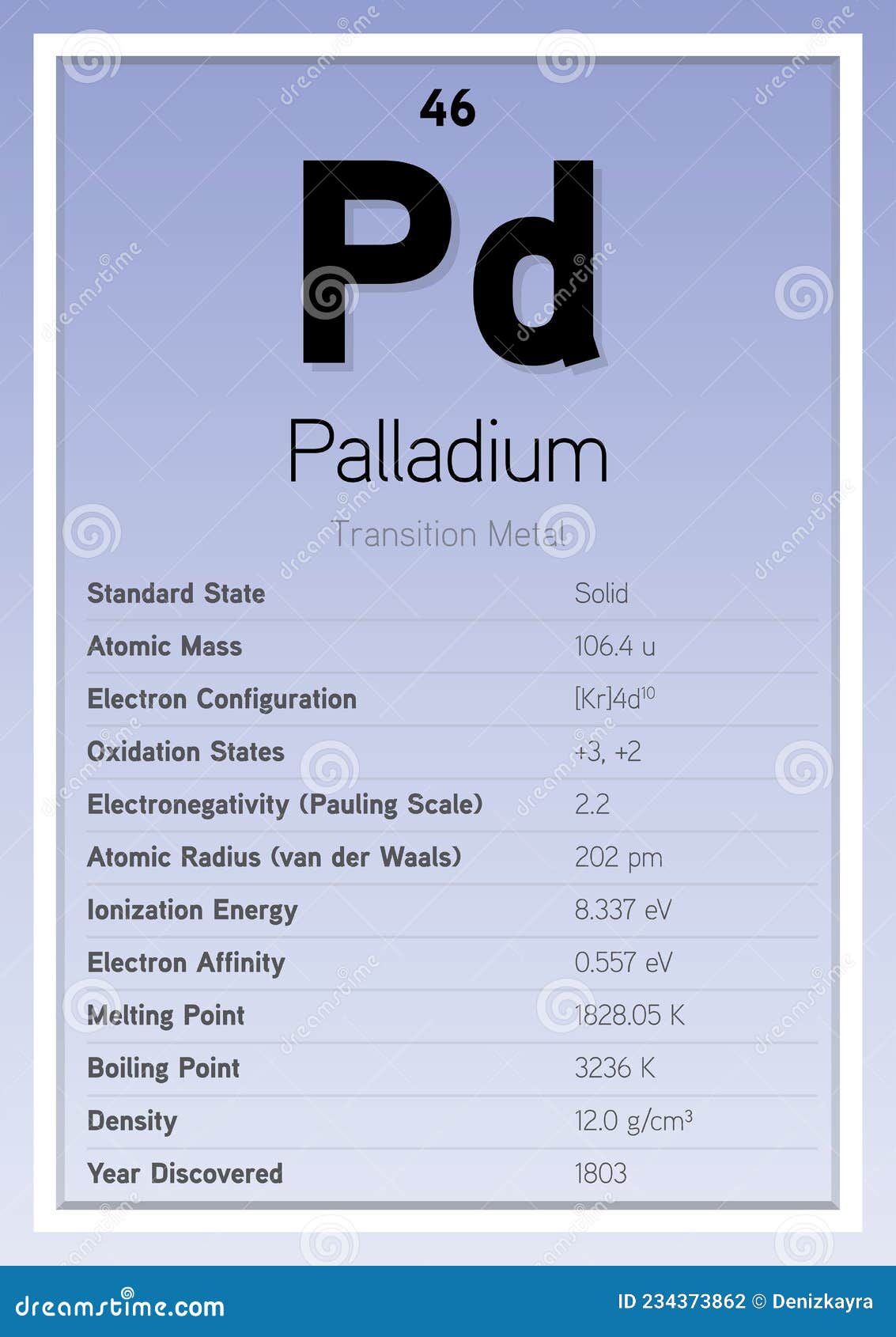
Palladium Periodic Table Elements Info Card (Layered Vector Illustration) Chemistry Education Stock Vector - Illustration of metals, erbium: 234373862
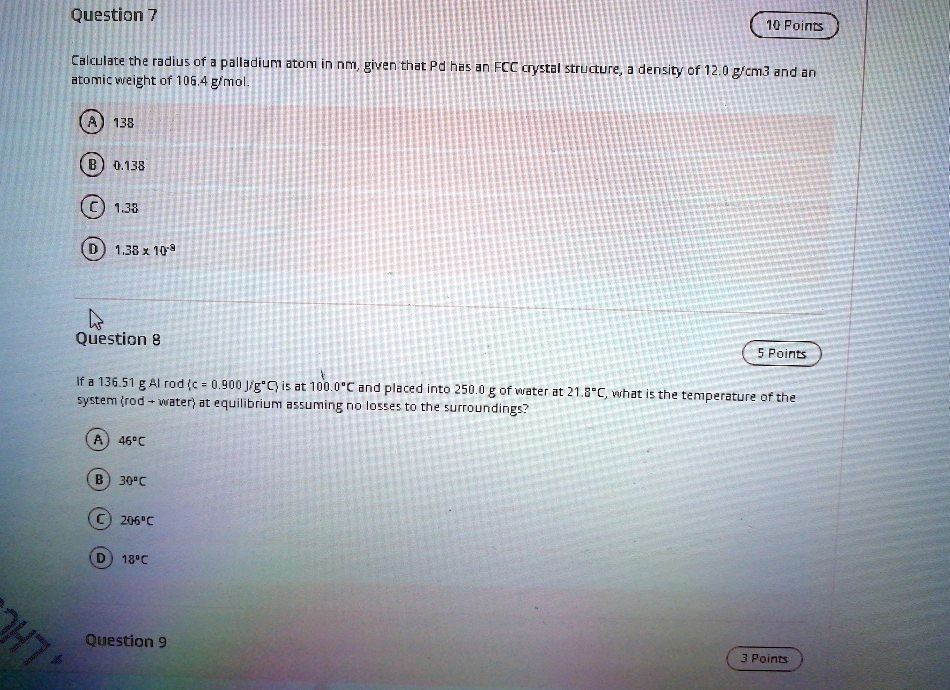
SOLVED: Question 7 10 Points Calculate the radius of palladium atom in nm, given that d has an FCC crystal structure, density of 12.0 gcm3 and an atomic weight of 106.4 gimol.